
On April 10, 2025, the U.S. Food and Drug Administration (FDA) officially launched a plan to reduce animal testing in preclinical safety studies, which has been incorporated into the Investigational New Drug (IND) application process. This new policy clearly states that mandatory animal testing requirements for monoclonal antibodies and other drugs will be gradually eliminated, and instead, "new methodologies" (NAMs) such as artificial intelligence (AI) computational models, human cell lines, organoids, and organ-on-a-chip systems will be used to obtain experimental data to replace traditional animal testing.
Dr. Martin A. Makary, Commissioner of the FDA, stated: "By relying on AI computational models, laboratory tests using human organ models, and real-world human data, we can not only accelerate the provision of safer treatment options for patients but also reduce drug development costs and drug prices, which has dual positive significance for public health and ethical development."
Looking back at the policy background, the United States passed the "FDA Modernization Act 2.0" in September 2022, which had already repealed the requirement for mandatory animal testing before human clinical trials of new drugs, laying the foundation for the application of non-animal alternative solutions such as AI models and organoid chips in IND applications. On this occasion, the FDA further promotes the implementation of the policy and simultaneously releases the "Roadmap for Reducing Animal Testing in Preclinical Safety Studies", which clarifies the implementation path for reducing animal testing through scientifically validated NAMs technologies (such as organ-on-a-chip systems, computational modeling, and advanced in vitro testing) and emphasizes the importance of multi-institutional collaborative promotion.
It is worth noting that in August 2022, the FDA approved the world's first new drug (clinical trial number: NCT04658472) with preclinical data obtained from "organ-on-a-chip" research to enter the clinical trial phase, marking the official recognition of non-animal alternative technologies. Against this backdrop, domestic and foreign enterprises have successively increased investment and exploration in technologies such as human organoids, organ-on-a-chips, and AI simulation tests. For example, MCE Corporation has signed a strategic cooperation agreement with the School of Pharmacy of Shanghai Jiao Tong University to carry out in-depth cooperation around "AI + organoids + drug research and development", and jointly build a national-level intelligent organoid drug research and development strategic platform to promote industrial technological upgrading.
1. Human Organoid Technology: A Key Bridge Connecting Traditional Culture and Animal Models
(1) Technological Development History
The concept of organoids was first proposed in 1997. It was not until 2009 that the team of Hans Clevers from the Netherlands Cancer Institute (NKI) successfully developed the first "complete" organoid model - the artificial intestinal organoid (Intestinal Organoid), marking a breakthrough in organoid research. In 2011, the team further constructed a colorectal cancer organoid model using tumor tissue from colorectal cancer patients, expanding the application of organoids to the field of oncology research. Currently, patient-derived organoid (PDO) models have become a hot direction in oncology research. In 2019, organoid research was featured on the cover of the international top academic journal Science and was selected as a major breakthrough of the year, demonstrating its important position in the field of life sciences.
(2) Technical Definition and Core Advantages
Organoids are a type of self-organizing cell culture that can simulate the structure and function of natural organs (such as liver organoids and intestinal organoids), and can be induced and generated from adult tissue stem cells or pluripotent stem cells. As a key bridge between traditional two-dimensional cell culture and animal models, organoids possess both experimental operability and the ability to capture biological complexity. Their core advantages have been verified through comparison with two-dimensional cell culture and animal research (see Table 1 for specific comparisons).
Table 1 Comparison of Organoid Culture, Two-Dimensional Cell Culture, and Animal Research[2]
| Evaluation Dimension | 2D Cell Culture | 3D Organoids | Animal Models |
| Physiological Characterization | Limited | Semi-physiological | Physiological |
| Vascularization and Immune System | No | No | Yes |
| High-Throughput Screening | Yes | Yes | No |
| Operability | Excellent | Good, but experimental variations may exist | Limited |
| Biobank | Yes | Yes, but limited to the cellular level | No |
| Genome Editing | Yes | Yes | Yes, but the generation of embryonic stem cells may be required |
| Organogenesis Modeling | Poor | Suitable for studying cell-cell communication and morphogenesis; reduced complexity | Yes, but often affected by the complex tissue environment |
| Human Development and Disease Modeling | Poor, due to over-simplified non-physiological conditions | Yes | Yes |
(3) Technical Preparation and Application Scenarios
There are two main approaches for the preparation of human organoids: one is to extract cells from normal or malignant primary tissues and induce their generation using the R-spondin method (a basic technology for intestinal organoid preparation); the other is to reprogram somatic cells into induced pluripotent stem cells and obtain organoids covering the three germ layers through directed differentiation technology.
Currently, organoid technology has been deeply integrated with cutting-edge technologies such as genome editing, single-cell genomics, live-cell imaging, and microfluidic technology, and is widely used in fields such as disease modeling, anti-cancer drug screening, drug toxicity identification, genetic testing, and cell therapy research and development. This technology not only provides a new perspective for analyzing organ development processes and disease pathogenesis but also significantly accelerates the research and development process of disease diagnosis technologies and treatment plans.
(4) Key Technical Operation Points
Sample Processing: Priority should be given to fresh surgical or biopsy samples. If cryopreservation is necessary, special cryopreservation solutions should be used; special reagents should be used during transportation to maintain cell viability; samples can be stored at 4°C for no more than 24 hours, and long-term storage should be placed in an environment of -80°C or liquid nitrogen (cell viability may decrease after resuscitation); samples should be washed with medium or PBS to remove blood, mucus, and debris, and ice-bath operation is recommended; for samples with high pollution risks such as intestinal and tumor samples, 1% penicillin-streptomycin (P/S) can be added to prevent pollution.
Tissue Digestion: The cleaned samples should be cut into appropriate sizes, and collagenase IV (mild type), trypsin (potent type), or commercial digestive solutions can be selected according to needs; the digestion status should be observed every 5 minutes to avoid over-digestion; serum-free stop solution should be used to terminate digestion to prevent interference from unknown components in fetal bovine serum (FBS).
Culture and Passage: Matrigel should be thawed in advance and operated on ice (it solidifies at room temperature); the cell seeding density should be optimized with reference to literature, and special medium should be used to provide substances required for growth and differentiation; after organoids are fully grown or mature, gentle pipetting or short-term digestion should be used for separation and passage; for cryopreserved organoids, cells with good viability after 2-3 passages should be selected, and the cryopreservation density should not be less than 2×105/mL; rapid thawing in a 37°C water bath is required during resuscitation to avoid cell damage caused by slow thawing.
Detection Methods: For cell viability detection, 3D & organoid ATP detection reagents and viability detection reagents can be used; for phenotypic identification, DAPI nuclear staining and specific marker recognition should be combined to achieve multi-dimensional analysis.
2. Organ-on-a-Chip System: A Cutting-Edge Technology for Microphysiological Environment Simulation
(1) Technological Development Background
With the approval of the NCT04658472 new drug clinical trial, the organ-on-a-chip technology based on the microphysiological system (MPS, which realizes multi-organ linkage through microfluidic technology) has gradually become the focus of the industry. As a miniaturized system in the field of bioengineering, the core of this technology is to culture cells or tissues in microfluidic chips, integrating multi-disciplinary technologies such as biology, materials science, and engineering. Relying on microfluidic equipment (such as microscopes and lens-free microscopes) and monitoring systems (such as actuators, sensors, and biosensors), it realizes real-time monitoring of cell growth and viability and precise regulation of the culture microenvironment.
(2) Technical Structure and Core Components
Organ-on-a-Chips (OOC) are mainly composed of three parts: first, microchannel structures, which are used to simulate the organ tissue environment and provide space for cell attachment, growth, and interaction; second, living cell systems, such as stem cells, which are cultured in microchannels or pore structures; third, bionic environment systems, which simulate the physiological environment of in vivo organs by precisely controlling nutrient supply, oxygen concentration, liquid flow, and other conditions. Taking the scaffold-free bone marrow chip as an example, its structure includes a glass layer for real-time microscopic observation and three polydimethylsiloxane (PDMS) layers that accommodate the upper cell culture microchamber, which is separated from the lower medium perfusion channel, and material exchange is realized through an intermediate porous membrane (see Figure 1 for the specific structure).
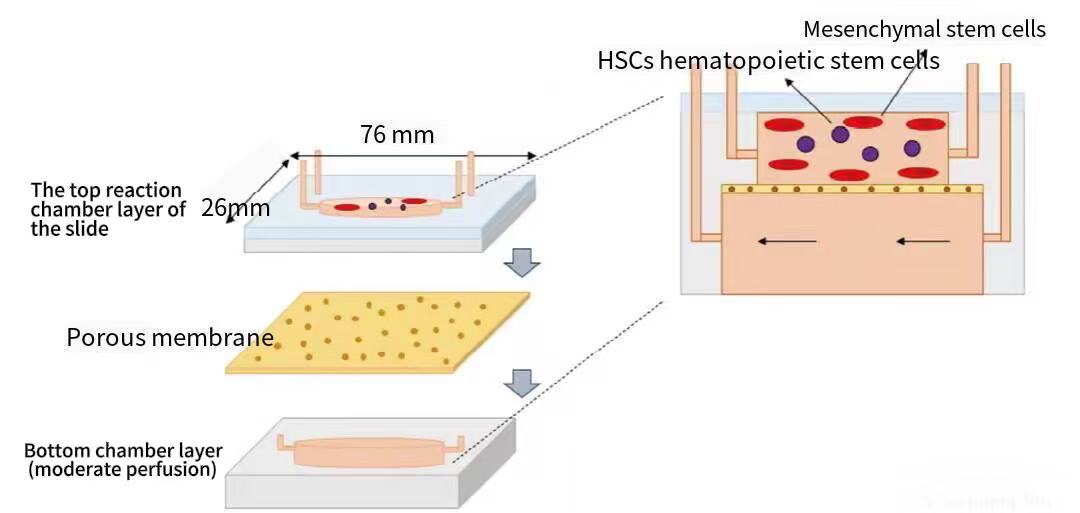
Figure 1 Schematic Cross-Section of a Scaffold-Free Bone Marrow Chip (Magnified 3D View) [5]
(Note: This structure includes a glass observation layer, three PDMS layers, an upper cell culture microchamber, a lower medium perfusion channel, and an intermediate porous membrane)
(3) Technical Advantages and Application Scenarios
Compared with organoid technology, organ-on-a-chip further integrates microfluidic, mechanical force, and multi-cell co-culture technologies, enabling more accurate simulation of the in vivo physiological environment. For example, the human liver chip can realize the co-culture of hepatocytes and non-parenchymal cells under perfusion conditions, exhibiting metabolic functions and response characteristics similar to those of the in vivo liver, and can capture human-specific biological effects that cannot be simulated by animal models.
Organ-on-a-chip technology has five core advantages: first, it reduces and ultimately replaces animal testing, can construct highly reproducible human models, supports high-throughput screening and testing, reduces research and development costs and time, and improves experimental efficiency; second, it supports personalized testing, and by integrating the patient's own cells, it can real-time monitor drug responses, effectiveness, and toxicity, and accurately identify biomarkers; third, it is suitable for medical testing scenarios, and with its small size and high degree of automation, it can be applied to point-of-care diagnosis to achieve decentralized testing; fourth, it has parallel testing capabilities, and multiple detection items can be integrated on the same chip, without relying on complex instruments; fifth, it facilitates drug delivery and toxicity testing, can construct human disease models, evaluate the targeted drug delivery effects of carriers such as nanoparticles, and simultaneously accurately detect drug efficacy and toxicity.
At present, the developed types of organ-on-a-chips include brain-on-a-chip, bone-on-a-chip, kidney-on-a-chip, lung-on-a-chip, pancreas-on-a-chip, heart-on-a-chip, stomach-on-a-chip, etc. Furthermore, the human-on-a-chip technology has been derived - which simulates the functions of multiple human organs by constructing microfluidic devices. Human-on-a-chip technology is more complex, requiring the integration of 2-10 organ modules, the design of complex microfluidic channels and precise processing systems (for automated scenarios), the culture of cells from different organ sources in multiple chambers, and the simulation of blood circulation through circulating medium to realize multi-organ physiology research. It can not only predict the impact of drugs on target organs but also conduct in-depth analysis of the potential side effects of drugs on other organs.
(4) Technical Application Case
In April 2022, John W. Rumsey and others published a research result titled "Classical Complement Pathway Inhibition in a 'Human-On-A-Chip' Model of Autoimmune Demyelinating Neuropathies" in the journal Advanced Therapeutics, which for the first time constructed a human-on-a-chip (HoaC) electrical conduction model for the study of chronic inflammatory demyelinating polyneuropathy (CIDP) and multifocal motor neuropathy (MMN) (see Figure 2 for the specific structure).
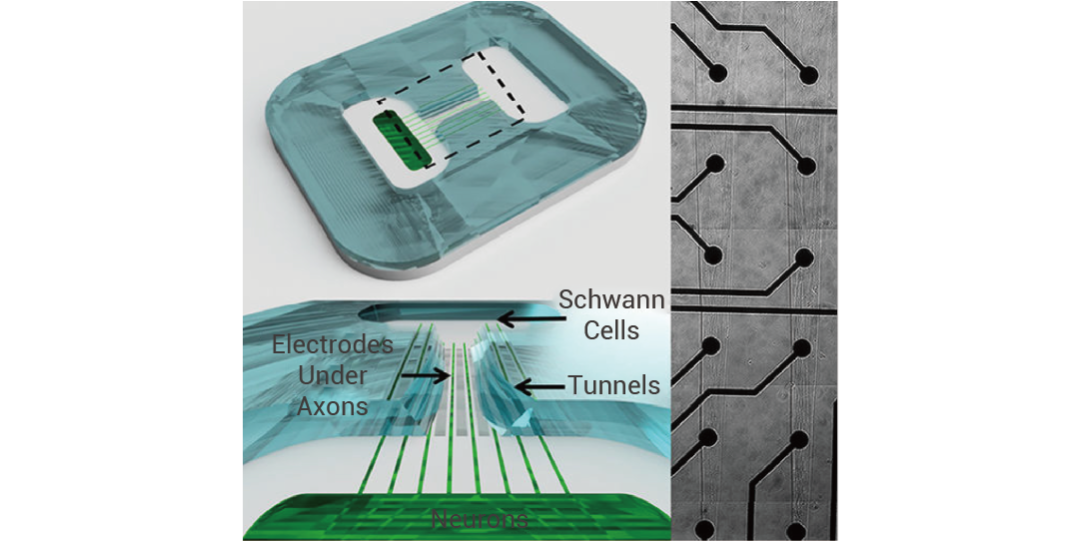
Figure 2 A New In Vitro Human-On-A-Chip System [6]
(Note: This system includes motor neurons derived from human induced pluripotent stem cells (iPSCs), human Schwann cells, and a microelectrode array (MEA). The MEA is equipped with channels to guide axon growth to the electrodes)
This model guides axon growth through the microelectrode array. After adding patient serum, it exhibits a disease phenotype of decreased motor neuron action potential frequency and slowed conduction velocity; however, after treatment with TNT005 (a murine monoclonal antibody (mAb) that inhibits C1s, a key protease in the classical complement pathway), the serum-induced complement deposition and functional defects were significantly improved. The data generated by this model provided key support for the preclinical C1s inhibition effect test of the NCT04658472 clinical trial, and ultimately helped this new drug pass FDA approval and enter the clinical trial phase.
3. Advanced Computer Simulation Technology: A Digital Tool for Drug Behavior Prediction
In the newly released roadmap, the FDA clearly encourages developers to use computer modeling and AI technologies to predict drug behavior. Currently, the core computer simulation tools mainly include the following four categories:
(1) Physiologically Based Pharmacokinetic (PBPK) Modeling
PBPK models use species-specific physiological parameters to mathematically simulate the absorption, distribution, metabolism, and excretion (ADME) processes of drugs, which can accurately predict the pharmacokinetic characteristics of drugs in different species and provide a scientific basis for clinical dose design and inter-species dose conversion.
(2) Machine Learning and AI Prediction Models
Machine learning algorithms can be trained based on drug sequence features, structural motifs, and known clinical results. Currently developed models can predict the immunogenicity of monoclonal antibodies by analyzing the amino acid sequence of the antibody variable region, providing a fast and efficient detection method for the immunological safety evaluation of antibody drugs.
(3) Quantitative Systems Pharmacology (QSP) and Biological Pathway Modeling
QSP models integrate computational biology and pharmacology theories to simulate the interaction between drugs and complex human biological networks. For example, in the research of autoimmune diseases, QSP models can simulate the regulatory mechanism of antibodies on inflammatory pathways, predict the effective dose range of drugs and potential toxicity risks (such as excessive suppression of the immune system), and significantly reduce the reliance on animal disease models by constructing a "virtual human body" to test different experimental scenarios.
(4) Bioinformatics and In Silico Off-Target Screening
Relying on human protein databases and AI technologies, it is possible to screen for potential unintended targets in drug sequences (such as cross-reactivity with human tissues). This technology identifies potential safety hazards by analyzing the binding potential of drugs to similar epitopes in the human proteome, replacing traditional animal tissue cross-reactivity studies and extensive receptor binding panel detection, and improving the efficiency and accuracy of drug safety evaluation.
In summary, as an important part of natural medical models (NAMs), computer simulation technology can predict human-related experimental results through data integration and modeling analysis, and has become an important supplement and alternative tool for animal research, providing key support for the digital and precise development of drug research and development.
[1] XIAO Yi, et al. Research progress and future perspectives of tumor organoid[J]. China Oncology, 2024, 34(8): 763-776
[2] Li M, et al. Organoids - Preclinical Models of Human Disease. N Engl J Med. 2019 Feb 7;380(6):569-579.
[3] Sato T, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009 May 14;459(7244):262-5
[4] Jacob F, et al.A Patient-Derived Glioblastoma Organoid Model and Biobank Recapitulates Inter- and Intra-tumoral Heterogeneity. Cell. 2020 Jan 9;180(1):188-204.e22.
[5] Obeid PJ, et al. Organ-On-A-Chip Devices: Technology Progress and Challenges. Chembiochem. 2024 Dec 2;25(23):e202400580.
[6] Rumsey JW, et al. Classical Complement Pathway Inhibition in a "Human-On-A-Chip" Model of Autoimmune Demyelinating Neuropathies. Adv Ther (Weinh). 2022 Jun;5(6):2200030.
[7] Roadmap to Reducing Animal Testing in Preclinical Safety Studies.










